Tamed energy - explosion directly in the electrolyzer
As early as Year 8 in the oxyhydrogen experiment in physics lessons, it became clear that hydrogen and oxygen can form a very intimate and noisy bond.
So if hydrogen and oxygen are close together, as is the case in both fuel cells and electrolysers, the risk scenario should also be considered if the two reaction partners come into unwanted contact.
Basics
Before we continue with the actual explosion, we should clarify a few things for a better understanding.
Detonation and deflagration
The difference between detonation and deflagration lies mainly in the speed at which a chemical reaction (such as an explosion) propagates:
Deflagration: Here the reaction spreads relatively slowly, usually at a speed below the speed of sound in the material concerned. A good example is fireworks or the burning of wood. The flame front moves “slowly” through the material and the increase in pressure is comparatively low.
Detonation: In a detonation, the reaction spreads extremely quickly, often faster than the speed of sound in the material. This leads to a very rapid increase in pressure and a shock wave that releases a lot of energy. An example of this is the explosion of explosives.
To summarize: Deflagration is a slow combustion (below the speed of sound). Detonation is a very fast explosion (above the speed of sound) with a strong shock wave.
Both are possible in the reaction of oxyhydrogen gas and depend on the boundary conditions.
Mach Number
The Mach number indicates how fast an object is compared to the speed of sound. At Mach 1 it flies as fast as sound, at Mach 2 twice as fast. Values below Mach 1 are slower than sound (subsonic), values above are faster (supersonic).
Explosion front
An explosion front is the front boundary of a propagating explosion. It is the area where the pressure and temperature increase suddenly and rapidly as the reaction propagates through the material or environment. The explosion front often travels in the form of a shock wave and carries the explosive force and pressure wave with it.
Difference between electrolyzer and fuel cell
In an electrolyser, electricity is used to break down water into its components, oxygen and hydrogen, so that the hydrogen can be used as an energy source. In the fuel cell, electricity and water are generated from the controlled combination of hydrogen and oxygen. It is therefore exactly the reverse process.
A little more precisely:
An electrolyser is a device that breaks down water (H₂O) into its components hydrogen (H₂) and oxygen (O₂) using an electric current. Current flows through two electrodes, an anode and a cathode, producing hydrogen at the cathode and oxygen at the anode.
A fuel cell consists of two electrodes, an anode and a cathode, which are separated by an electrolyte membrane. Hydrogen (H₂) is split into protons and electrons at the anode, with the protons traveling through the membrane to the cathode, while the electrons pass through an external circuit and generate electricity. At the cathode, the protons and electrons react with oxygen (O₂) and form water (H₂O) as a waste product.
Fuel cells and electrolysers therefore have a similar structure in principle. In order to be as compact and powerful as possible, cells consisting of anode and cathode plates are stacked on top of each other and braced together via so-called end plates.
Risk
As long as the stack is tight and hydrogen and oxygen are separated from each other, everything is fine. But what happens if a cell is defective, e.g. if a membrane ruptures and hydrogen and oxygen mix unintentionally?
Then a local explosion can occur, which can also destroy neighboring cells. In extreme cases, all the hydrogen finds its beloved reaction partner oxygen and enters into an exothermic reaction, which already impressed us at school, only a little more strongly.
For the sake of simplicity, we will now look at an electrolyser.
Test
As part of risk assessments, the behavior of electrolysers in the event of an explosion must be verified in tests. These tests are very complex and expensive, especially as they usually result in a destroyed electrolyzer. The test in practice is that the system or parts of the system are flooded with an ignitable hydrogen-oxygen mixture and accesses to the outside are closed and encapsulated. Ignition takes place via an ignition source with a spark.
But what happens if the experiment fails or the effects cannot be sufficiently clarified? Then simulation - and therefore Merkle CAE Solutions - can help! The advantage here is that the computer is not destroyed and virtual prototypes cost comparatively little money.
Simulation
The question for fluid mechanics and reaction kinetics is:
- What pressures and temperatures occur during an explosion?
With the help of simulation at Merkle CAE Solutions, a wide variety of scenarios can be investigated, be it an explosion in one cell, in several cells or in the entire electrolyser.
Different compositions of the mixture can also be considered in this way:
Substoichiometric, stoichiometric and superstoichiometric.
Stoichiometric means that each oxygen atom has two hydrogen partners to react with. Almost like in dance school... where everyone only has one partner.
Understoichiometric means excess hydrogen, overstoichiometric means too little hydrogen. In both cases, the dance floor and therefore the crowds become smaller. Unfortunately, the comparison in our LGBTQ+ society is also not valid here. But the chemistry here is tough.
The stoichiometric mixture is therefore critical, as the entire volume is filled with an explosive mixture and this is where the explosion energy is greatest.
The questions for the structural simulation and containment are:
Does the system remain sealed in the various scenarios?
Does the explosion in one cell affect other cells, for example?
Will the structure blow up in your face or could someone be injured?
Under no circumstances may an ignitable mixture be be released into the environment.

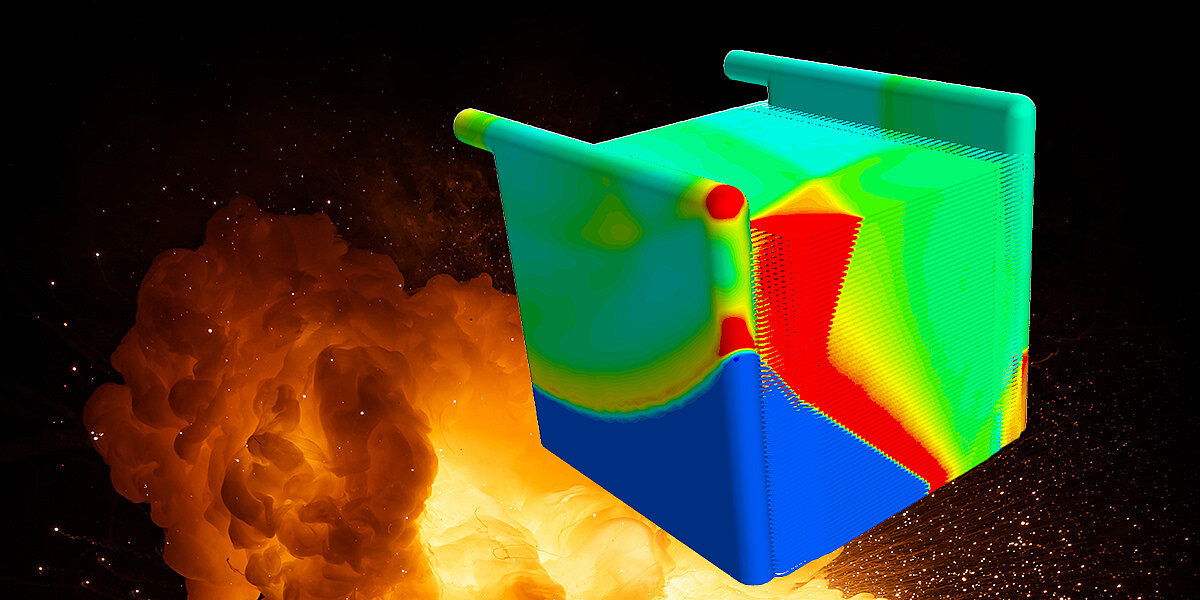
Results of the flow simulation
The presentation of the results of the explosion in the calculation at Merkle CAE Solutions shows that the pressure wave travels through the cells and the entire electrolyser.
The temperature near the wall is around 2400 °C and the pressure wave has a peak pressure of just over 100 bar.
The propagation speed of the detonation front is Mach 0.75 and is therefore still in the subsonic range. This is therefore a... deflagration.







This data can now be used in a structural-mechanical FEM calculation to check whether the strength is sufficient. This is usually the end of the calculation, but there is no reason not to consider the failure of the entire housing with the determined pressure and temperature boundary conditions in a crash model. The requirement in practice, however, is not how it fails, but what has to be done constructively so that it does not fail. Unfortunately, this is the case from a calculation point of view, because I find the failure more exciting... But that's just my personal opinion. 😊
And now the whole thing dynamically in the video:
We at Merkle CAE Solutions are also happy to support you with our unique know-how in the design of electrolyzers or fuel cells. We look forward to your inquiries.
Yours, Stefan Merkle
PS: I really like hydrogen as an energy carrier because it has a high energy density.